Automated Software Validation for Life Sciences
- Accelerate your Software Time-to-Value
- Reduce Your Compliance and Cybersecurity Risks

The Problem with Traditional Validation
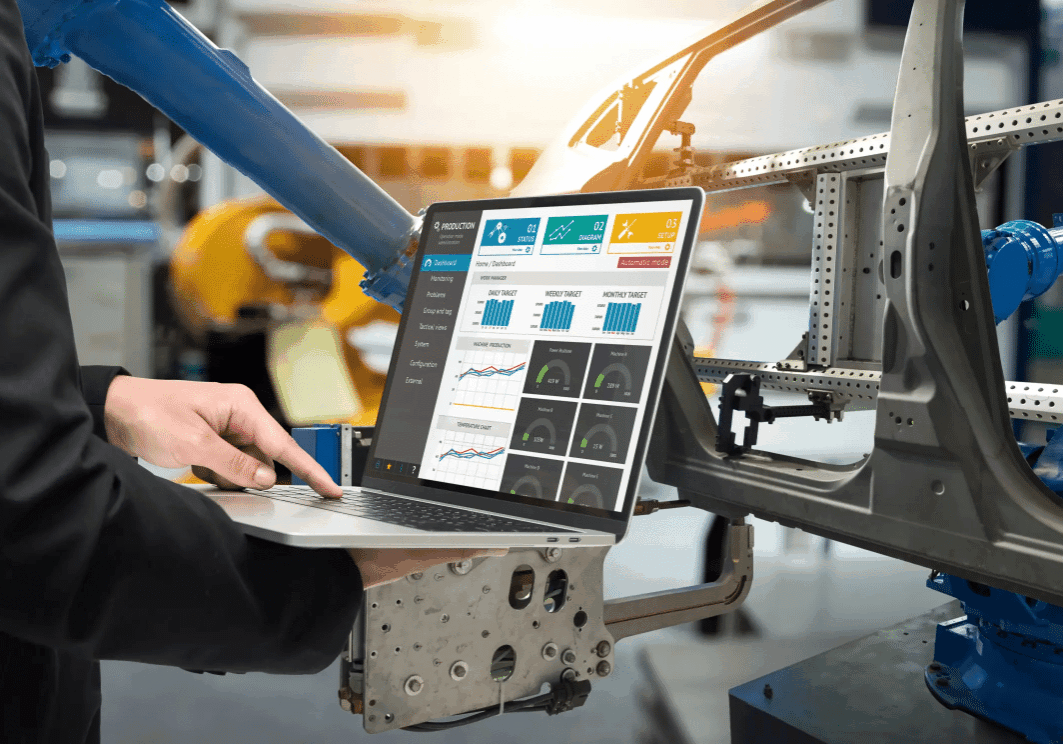
Manual validation methods in life sciences are slow, error-prone, and can’t keep up with evolving regulations. Critical systems like QMS, LIMS, MES, and ERP require frequent updates—putting pressure on teams to validate and maintain compliance without delays.
- Time-Consuming – Manual validation testing and high volume of document creation drag out validation cycles
- Error-Prone – Increased risk of mistakes, rework, and audit findings
- Hard to Scale – Frequent updates overwhelm internal teams
Power Your Validation Plan with Automation
SIMCO automates the most time-consuming phase of validation —test execution. Our services-based solution allows the creation & execution of test scripts aligned with your requirements and risk assessments, delivering faster results and cleaner compliance.
- Automated Execution – Runs test cases based on your validation plan
- Structured Results – Generates clear, audit-ready documentation
- Built for Reuse – Simplifies future updates with reusable script executions
- Track project progress - Track every stage of your validation document creation & Test Execution phases

Key Benefits of Automated Software Validation
SIMCO’s solution helps life sciences teams cut validation time by up to 93%, reduce costs, and stay audit-ready — all while meeting regulatory standards. Automate the entire validation lifecycle, from documentation to Performance Qualification (PQ) test execution, so you can focus on innovation, not paperwork.
- 93% Faster Validation Test Execution & 14x More Value - Eliminates manual effort, errors and rework
- Enhance Cybersecurity – Maintain a secure software environment with timely and automated updates across your ecosystem
- Expert PQ Support – Validate customized workflows with confidence
- Undisputed Audit Preparedness – Get complete, standards-compliant documentation every time
Validation You Can Trust. Proof You Can Show
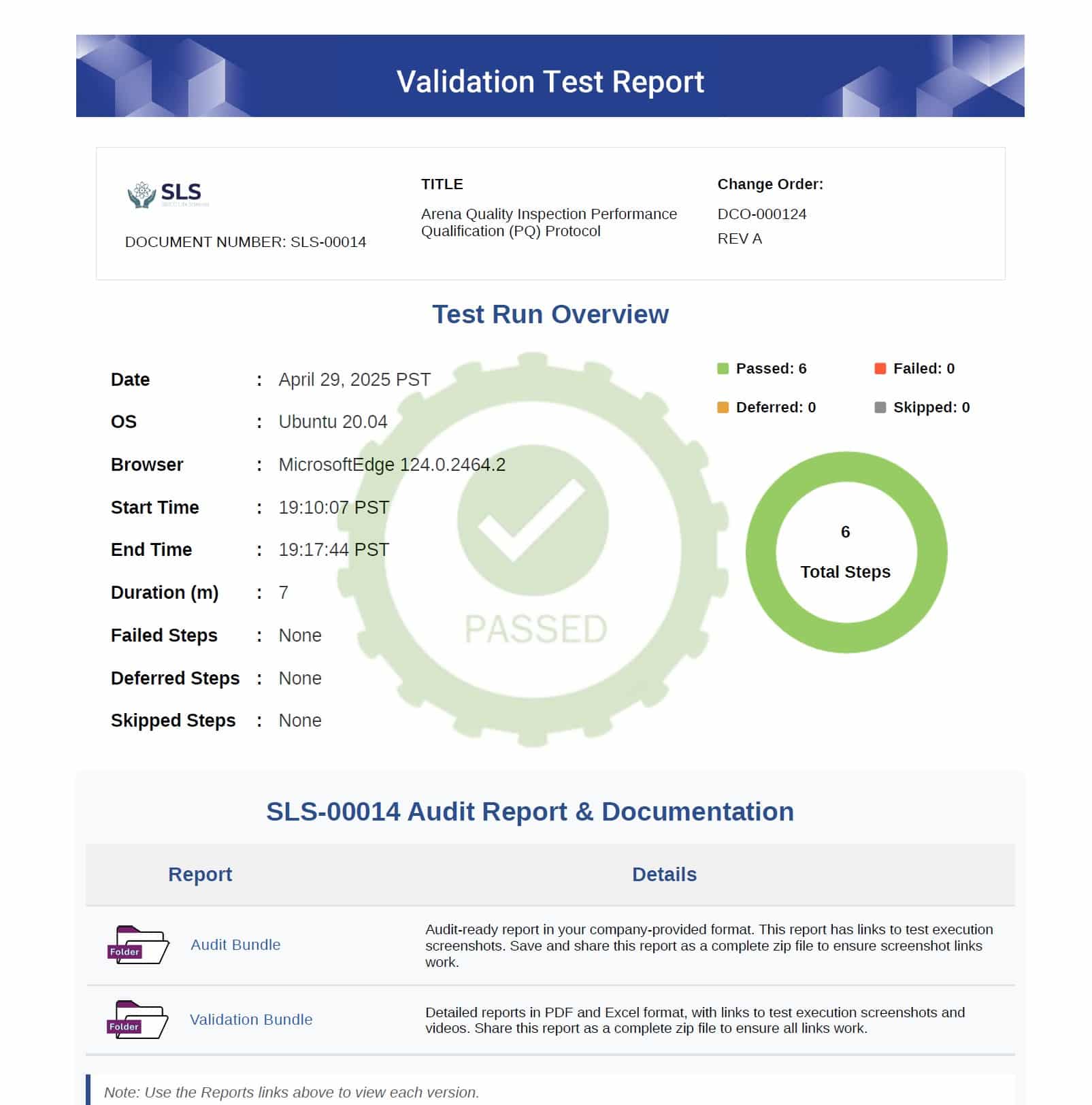
SIMCO’s Automated Software Validation delivers fast, compliant validation with clear, audit-ready documentation. Every test cycle generates structured records to satisfy both regulators and internal quality teams—giving you confidence at every step.
- Audit-Ready Reports – Summary results with digital signatures for fast inspections
- Detailed Test Evidence – Step-by-step logs with screenshots and video for full traceability
- Reusable Scripts – Pre-built automation ready for future updates and re-validation

Supports leading Software Applications
Run Automated validation tests for PQ workflows for the top industry SaaS software applications including QMS, ERP, LIMS, CRM, Document Management Systems, and many more. Ensure faster, error-free validation cycles with test libraries tailored to each application.
Expert Validation Documentation, Done for You
Beyond automated test case execution, SIMCO delivers complete validation documentation services—crafted by industry experts to meet regulatory standards. From planning to traceability, we help you build a compliant, audit-ready validation package with confidence.
We develop:
- User Requirements Specifications (URS)
- Validation Plan & Risk Assessment
- Test Cases
- Traceability Matrices