Compliance Module
FDA Compliance for Life Science Organizations
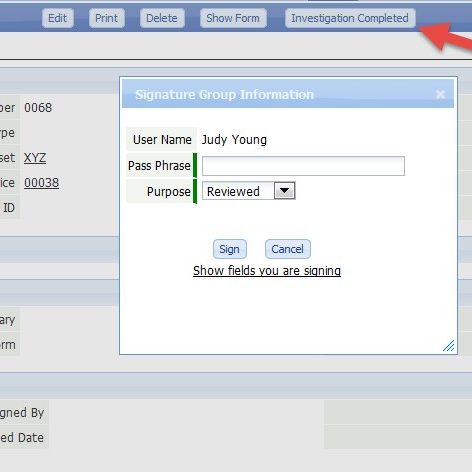
CERDAAC’s Compliance Module is an optional feature set for CERDAAC SIMCO Manager or Service Manager that helps life science companies comply with FDA guidelines.
Compliance Module complies with the industry’s most stringent quality guidelines and is validated to cover FDA 21CFR Part 11 (Electronic Records, Electronic Signatures) and relevant Part 820 (Quality System Regulation) requirements.
Its advanced capabilities and features include:
- Validated software with annual audit support
- FDA compliant electronic digital signatures
- Automated calibration interval adjustment
- Chain-of-command alerts and reports to enforce on-time compliance
To receive a datasheet or online demonstration of CERDAAC Compliance Module, please contact us.
For CERDAAC software support, call 866-681-6698.

